Yes, please!
When it comes to regulatory guidance for ad promo, the answers lie in the details. But no one wants to hunt through and compare thousands of pages of FDA guidances, warning letters, and guidelines. Now, with Margo, you don’t have to.
Meet

Margo is an AI search assistant that instantly answers marketers’ and reviewers’ marketing compliance questions. Every answer links to its sources, drawing from FDA, FTC, CCC, and your company’s governance documents. Put the single source of truth for compliant marketing at your fingertips.
- FDA’s complete Ad Promo guidances
- Your guidelines, standards, policies, and SOPs
- The Center for Communication Compliance’s (CCC) complete elearning library, exclusive to Margo
- Instant, specific answers linked to their sources
- Click-to-share and conversation storage
- Tested and trained by pharmaceutical marketing regulatory experts
- Built on OpenAI, ML, and NLP technologies
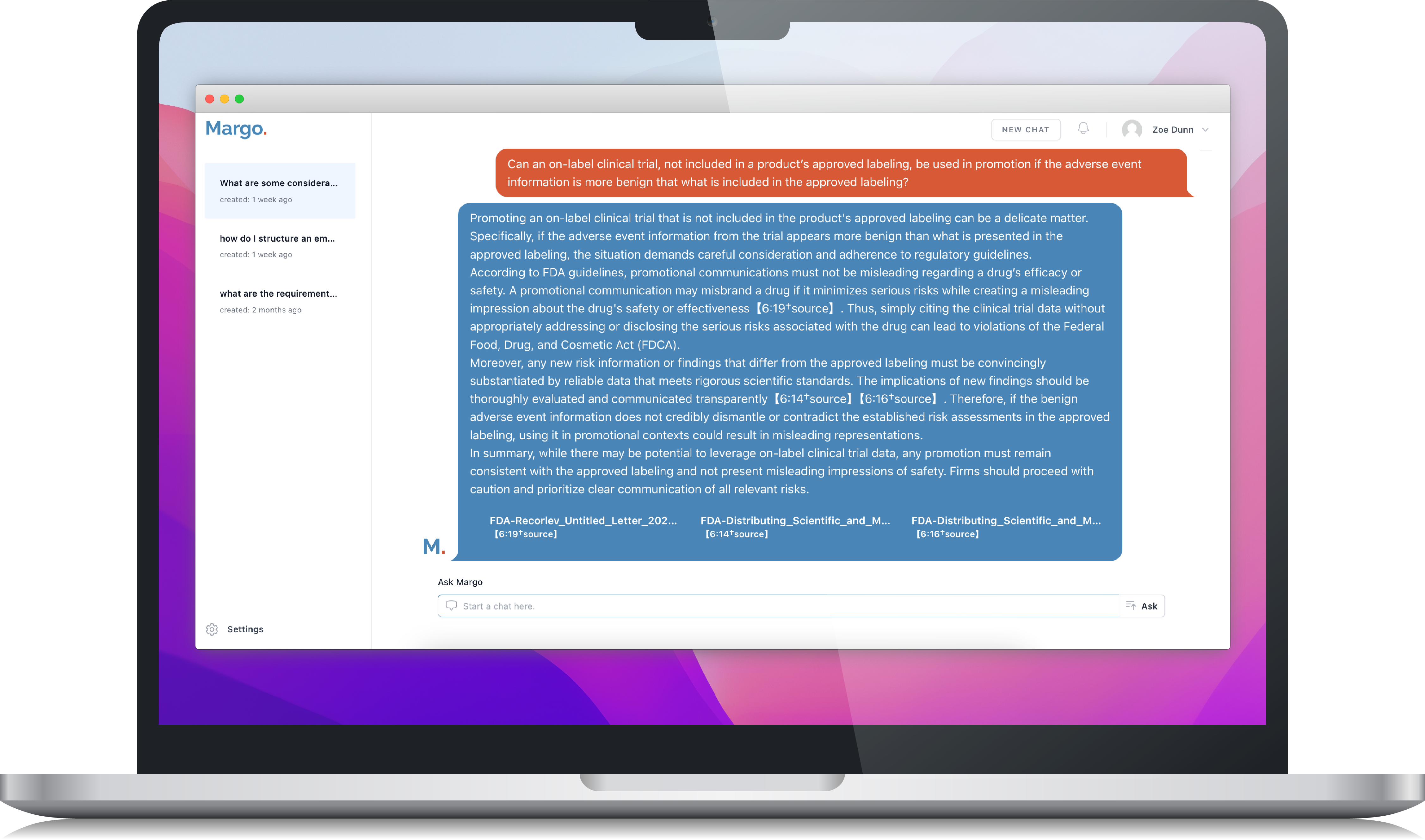
Margo is an AI search assistant that instantly answers marketers’ and reviewers’ marketing compliance questions. Every answer links to its sources, drawing from FDA, FTC, CCC, and your company’s governance documents. Put the single source of truth for compliant marketing at your fingertips.
- FDA’s complete Ad Promo guidances
- Your guidelines, standards, policies, and SOPs
- The Center for Communication Compliance’s (CCC) complete elearning library, exclusive to Margo
- Instant, specific answers linked to their sources
- Click-to-share and conversation storage
- Tested and trained by pharmaceutical marketing regulatory experts
- Built on OpenAI, ML, and NLP technologies

Not just more efficient, but smarter.
Margo empowers Marketing, Agencies, Marketing Operations, and Promotional Review to instantly find the information they need to create compliant marketing. Use Margo to inform concepts, guide creative execution, resolve debate in review meetings, and amend or write guidelines. The more you use Margo, the smarter you become.
- Instant access to compliance information drives guidelines usage
- Grows team’s regulatory knowledge
- Fosters marketing and regulatory collaboration
- Prevents compliance errors
- Unlocks creative and MLR efficiency
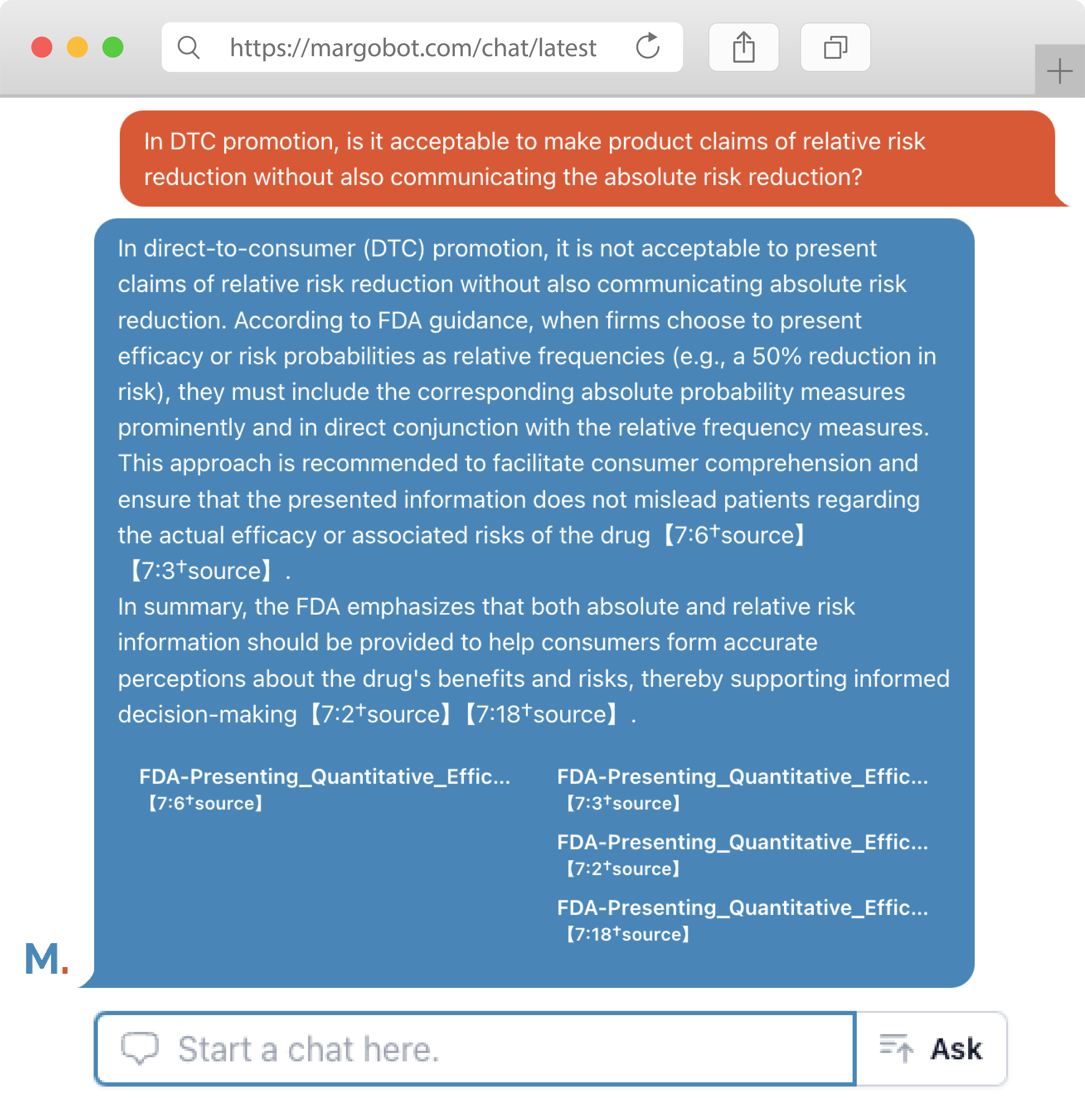
Imagine marketers coming to the table with materials that are already compliant with regulatory-approved guidelines—with citations at their fingertips. Margo just may become every marketing and PRC team’s favorite new ‘colleague.’
– Josie Waters
Consumer and Patient Marketing Director
The greatest challenge in following the rules is knowing the rules. Quick access to the right information means you can create compliant materials the first time around. You’ll save time and money, get to market faster, and protect your company from exposure to liability. Margo makes a difference.
– Zoë Dunn
President and CEO, Hale Advisors
FAQ.
How does Margo work?
Compliant materials are uploaded to Margo’s database. These include Margo’s base package of FDA guidances plus your regulatory-approved company guidelines or other marketing governance materials. Users can ask Margo questions related to these materials and receive detailed responses, along with links to the sources for reference.
Can Margo work with other sources like brand standards, message maps, or process documents?
Yes, Margo works with any document uploaded to your vector file, but we recommend static documents that require infrequent updates. This helps ensure the repository remains as current and accurate as possible.
Does Margo understand multiple languages?
Yes, Margo can understand and respond in all major languages.
At what points in the content creation and review journey do teams use Margo?
Margo can assist all functional areas and agency partners throughout the process. Use Margo to inform concepts, guide creative execution, resolve debate in review meetings, and amend or write new guidelines.
How does Margo handle aggregation and association errors?
Margo gives users citations and links to each answer’s source document(s) for validation, fact-checking, and more information.
How does Margo ensure privacy and security?
Margo is developed and maintained with best-in-class coding standards to ensure privacy and security. All client information is encrypted at rest and secured during transit. Margo does not collect any other sensitive or user tracking information.
How is Margo priced?
Margo operates on a concurrent user licensing model. Instead of requiring a license for every potential user, the license is shared across a pool of users in your organization. This model provides simplicity, flexibility, scaleability, and cost savings. Contact us for pricing.



